Impact of tunable 2-(1H-indol-3-yl)acetonitrile based fluorophores towards optical, thermal and electroluminescence properties†
Abstract
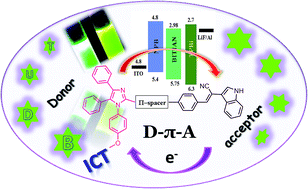
Herein, we have synthesized 4,5-diphenyl-1H-imidazole and 2-(1H-indol-3-yl)acetonitrile based donor–π–acceptor fluorophores and studied their optical, thermal, electroluminescence properties. Both the fluorophores exhibit high fluorescence quantum yield (Φf = <0.6) and good thermal stability (Td10 = <300 °C), and could be excellent candidates for OLED applications. Moreover, the ground and excited state properties of the compounds were analysed in various solvents with different polarities. The geometric and electronic structures of the fluorophores in the ground and excited states have been studied using density functional theory (DFT) and time-dependent density functional theory (TDDFT) methods. The absorption of BIPIAN and BITIAN in various solvents corresponds to S0 → S1 transitions and the most intense bands with respect to the higher oscillator strengths are mainly contributed by HOMO → LUMO transition. Significantly, the vacuum deposited non-doped OLED device was fabricated using BITIAN as an emitter, and the device shows electroluminescence (EL) at 564 nm, maximum current efficiency (CE) 0.687 cd A−1 and a maximum external quantum efficiency (EQE) of 0.24%.


 Please wait while we load your content...
Please wait while we load your content...