The synthesis of HMF-based α-amino phosphonates via one-pot Kabachnik–Fields reaction†
Abstract
The first use of biomass-derived HMF in the one-pot Kabachnik–Fields reaction is reported here. A wide range of furan-based α-amino phosphonates were prepared in moderate to excellent yields under mild, effective and environmentally-benign conditions: iodine as a non-metal catalyst, biobased 2-MeTHF as the solvent and room or moderate temperature. The hydroxymethyl group of HMF persists in the Kabachnik–Fields products, widening the scope of further modification and derivatization compared to those arising from furfural. Issues involving the diastereoselectivity and double Kabachnik–Fields condensation were also faced.
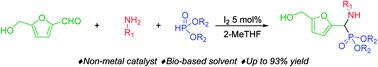


 Please wait while we load your content...
Please wait while we load your content...