On the direct use of CO2 in multicomponent reactions: introducing the Passerini four component reaction†
Abstract
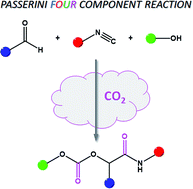
We introduce a novel isocyanide-based multicomponent reaction, the Passerini four component reaction (P-4CR), by replacing the carboxylic acid component of a conventional Passerini three component reaction (P-3CR) with an alcohol and CO2. Key to this approach is the use of a switchable solvent system, allowing the synthesis of a variety of α-carbonate-amides. The reaction was first investigated and optimized using butanol, isobutyraldehyde, tert-butyl isocyanide and CO2. Parameters investigated included the effect of reactant equivalents, reactant concentration, solvent, catalyst, catalyst concentration and CO2 pressure. Of the other parameters, the purity of the aldehyde and its tendency to oxidize was one of the most critical parameters for a successful P-4CR. After optimization, a total of twelve (12) P-4CR compounds were synthesized with conversions ranging between 16 and 82% and isolated yields between 18 and 43%. Their structures were confirmed via 1H and 13C NMR, FT-IR and high resolution mass spectrometry (ESI-MS). In addition, three (3) hydrolysis products of P-4CR (α-hydroxyl-amides) were successfully isolated with yields between 23 and 63% and fully characterized (1H, 13C NMR, FT-IR and ESI-MS) as well.


 Please wait while we load your content...
Please wait while we load your content...