Removal of Cd(ii) and Pb(ii) ions from natural water using a low-cost synthetic mineral: behavior and mechanisms
Abstract
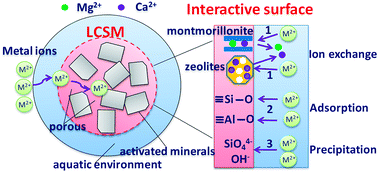
A low-cost synthetic mineral (LCSM) was prepared by mechanochemical treatment of a solid-state mixture containing potassium feldspar, wollastonite, gypsum, limestone and dolomite powder at a molar ration of 1 : 1 : 1 : 6 : 3 and hydration process. The predominant activated mineralogical compositions of LCSM are gehlenite, montmorillonite and zeolites (laumontite and gismondine). The Cd(II) and Pb(II) ion removal behavior of LCSM from natural water was evaluated in batch mode as a function of contact time, pH, temperature, adsorbent dosage and concentration of initial metals. The results indicated that the adsorption process was pH dependent, endothermic and spontaneous. Meanwhile, the adsorption experiment data follows the Freundlich isotherm and the kinetic data best fit the pseudo-second order kinetic model. The maximum adsorption capacity obtained from the Freundlich isotherm at 25 °C was 32.8 mg g−1 for Cd(II) and 268 mg g−1 for Pb(II) ions, showing much higher removal capacity than the relevant previous studies. The metal ion removal by LCSM mainly occurs by an ion exchange mechanism, followed by precipitation and adsorption. Further, the adsorbed Cd(II) and Pb(II) on LCSM can hardly be desorbed at pH 3.0 and the desorption rates were even below 20% at pH 2.0, which indicates the excellent stability of LCSM and the heavy metals adsorbed are difficult to release into natural water. Therefore, despite its poor regenerability, LCSM can still be a good alternative adsorbent for removing metal ions from natural water or a soil amendment used for heavy metal immobilization when considering the abundance of low-cost raw materials used for its preparation, its good chemical stability and the amounts of mineral nutrients it contains.


 Please wait while we load your content...
Please wait while we load your content...