Use of CuO encapsulated in mesoporous silica SBA-15 as a recycled catalyst for allylic C–H bond oxidation of cyclic olefins at room temperature†
Abstract
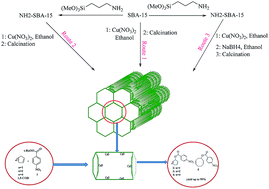
CuO nanoparticles were deposited on SBA-15 in three routes. These were metal loading on SBA-15 and calcination at 550 °C (Cu–SBA-15), metal loading on 3-aminopropyl-trimethoxysilane grafted SBA-15 and calcination at 550 °C (Cu–N–SBA-15), and metal loading on 3-aminopropyl-trimethoxysilane grafted SBA-15 and reduction by NaBH4 then calcination at 550 °C (Cu–B–N–SBA-15). These catalysts were characterized through powder X-ray diffraction (XRD), BET nitrogen adsorption–desorption methods, energy dispersive X-ray (EDX), atomic absorption spectroscopy (AAS), elemental mapping, scanning electron microscopy (SEM), and Fourier transform infrared (FT-IR) spectroscopy. The result of the heterogeneous catalysts being applied in the monometallic direct catalytic esterification of sp3 C–H bonds in cyclic olefins for the first time is reported, using tert-butyl 4-nitrobenzoperoxoate at room temperature. Allylic esters were obtained as the product of the C–H bond oxidation reaction in moderate to good yields (up to 99%) within reasonable reaction times. Also, the recovered catalyst (Cu–B–N–SBA-15) is applicable in the oxidation reaction for four times with little loss in reactivity and yield.

 Please wait while we load your content...
Please wait while we load your content...