Methyl and phenyl substituent effects on the catalytic behavior of NHC ruthenium complexes†
Abstract
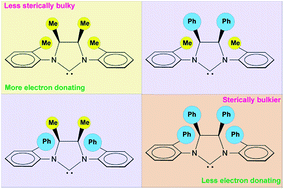
New second-generation ruthenium benzylidene and isopropoxybenzylidene catalysts bearing N-heterocyclic carbene (NHC) ligands with o-biphenyl groups at the N-atoms and syn methyl or phenyl groups on the backbone were obtained and their catalytic behaviors were compared to those of analogous N-o-tolyl catalysts in standard ring-closing metathesis (RCM) reactions. A pronounced difference in catalyst efficiency was observed depending on the nature of ortho-N-aryl substituents (methyl or phenyl). Notably, very impressive catalytic performances were exhibited by N-o-biphenyl complexes with a syn dimethyl backbone in the formation of di- and trisubstituted cycloalkenes. To rationalize catalytic results, methyl and phenyl substituent effects on the steric and electronic properties of NHC ligands were assessed through experimental and theoretical investigations involving ruthenium complexes as well as newly developed rhodium derivatives. Despite the different electron donor capacities of the examined carbenes, the steric differences shown by N-o-biphenyl and N-o-tolyl NHCs, although subtle, were found to be the key factor in addressing catalyst behavior.

 Please wait while we load your content...
Please wait while we load your content...