Understanding structural characteristics of out-of-register hIAPP amyloid proteins via molecular dynamics†
Abstract
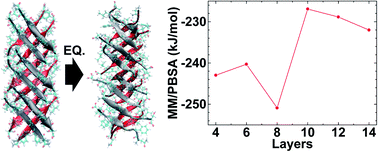
Amyloid oligomers are implicated in several neurodegenerative diseases; studies have shown oligomeric amyloids form fibrillary amyloids and have toxic effects on cell function. Several experimental and computational studies have investigated in-register amyloids and their characteristics. However, recently, out-of-register amyloid structures have been observed and their inherent weak structural stability exhibits higher toxicity under physiological conditions compared to that of in-register amyloids. Specifically, by varying the size of oligomeric hIAPP out-of-register structures from 4 layers to 20 layers, we successfully analyzed the structural characteristics of fibrillary out-of-register hIAPP; the critical structure size of out-of-register hIAPP is related to fibrillar growth from protofibrils. Through the structural analysis of out-of-register hIAPP, we shed light on the fibrillar growth mechanism of out-of-register hIAPP oligomer in detail.

 Please wait while we load your content...
Please wait while we load your content...