Tuning α-Fe2O3 nanotube arrays for the oxygen reduction reaction in alkaline media
Abstract
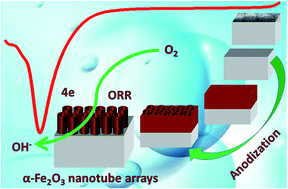
The oxygen reduction reaction plays a crucial role in alkaline fuel cells. Herein, an α-Fe2O3 nanotube array material was fabricated via a facile two-step electrochemical anodization method and employed as an efficient ORR catalyst in alkaline media. Due to its highly ordered open top architecture, the α-Fe2O3 nanotube array electrode exhibits excellent ORR catalytic activity with an onset potential of −0.39 V (vs. Hg/HgO) and high current density of 6.95 mA cm−2. Results show that the ORR exhibits quasi-reversible diffusion-controlled reaction characteristics and a well-defined four electron pathway. Moreover, as the crystalline structure transforms from α-Fe2O3 to Fe3O4 or as the alkaline concentration increases, the ORR activity is disturbed and the electron transfer number decreases. The as-prepared electrodes possess favorable alkaline tolerance as indicated from their chronoamperometric curves and Raman spectra. Accordingly, this novel α-Fe2O3 nanotube array material can be employed as efficient and low cost non-noble metal electrodes for electrochemical energy applications.

 Please wait while we load your content...
Please wait while we load your content...