Synthesis and biological activity evaluation of 20-epi-salinomycin and its 20-O-acyl derivatives†
Abstract
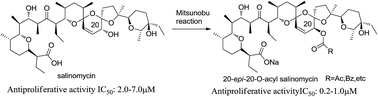
20-epi-Salinomycin and six 20-O-acylated analogs were synthesized and tested for their antiproliferative activity. Both the C1-protecting group of the salinomycin and the acidity of the substituted benzoic acid are crucial to the Mitsunobu conversion. 20-epi-Salinomycin showed similar antiproliferative activity as salinomycin, but its 20-O-acylated analogs were 2–10 times more potent. In addition, the 20-epi-20-O-acylated salinomycin derivative 9d and 9e had better selectivities than salinomycin between cancer and neuron cell lines. The spatial configuration of the C20-hydroxyl group has little influence on the activities, but the acyl groups cause an obvious difference by producing possible effects on the stability and permeability of the salinomycin–alkali metal ions complexes.

 Please wait while we load your content...
Please wait while we load your content...