Themoxidative stability and char formation mechanism for the introduction of CNTs and MoS2 into halogen-free flame retarding TPEE
Abstract
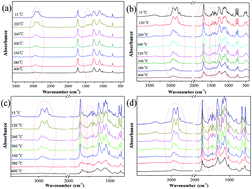
In this study, we give an insight into the char formation mechanism for the addition of CNTs and MoS2 into halogen-free flame retarding thermoplastic poly(ether ester) elastomers (TPEE). We used real-time Fourier transform infrared spectroscopy (FTIR) to analyze the change in the characteristic peaks of the formulations during the themoxidative process. Thermoxidative stability of the samples has been investigated by thermogravimetric analysis (TGA) under air atmosphere at a heating rate of 10 °C min−1. The catalyzation of carbon nanotubes (CNTs) during the thermo-oxidative process has been demonstrated. The pyrolysis products for different formulations have been studied by pyrolysis/gas chromatography/mass spectroscopy (pyrolysis/GC/MS). Variations in the composition of volatile decomposition products (Mn < 100 g mol−1) have been discussed. The morphology and the graphite degree of the residues remaining at high the temperature have been studied by scanning electron microscopy (SEM) and Raman spectroscopy, respectively. We found that the Mo element can effectively catalyze the char forming process through a cycloaddition interaction of volatile decomposition products. TPEE/P–N/CNTs/MoS2 exhibited the best stable-char structure for the combination of the bone structure of CNTs and the cycloaddition reaction of the pyrolysis gas products with low molecular weight.

 Please wait while we load your content...
Please wait while we load your content...