Iodine-mediated synthesis of benzopyridothiazines via tandem C–H thiolation and amination†
Abstract
The first example of an iodine-mediated tandem C–H thiolation/amination strategy for the synthesis of benzopyridothiazines by the reaction of secondary amines with pyridine disulfides is described.
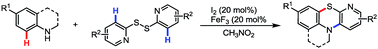

 Please wait while we load your content...
Please wait while we load your content...