Structure activity related, mechanistic, and modeling studies of gallotannins containing a glucitol-core and α-glucosidase†
Abstract
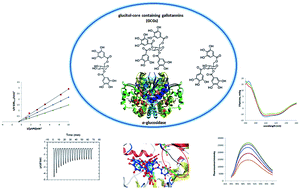
Gallotannins containing a glucitol core, which are only produced by members of the maple (Acer) genus, are more potent α-glucosidase inhibitors than the clinical drug, acarbose. While this activity is influenced by the number of substituents on the glucitol core (e.g. more galloyl groups leads to increased activity), the mechanisms of inhibitory action are not known. Herein, we investigated ligand–enzyme interactions and binding mechanisms of a series of ‘glucitol-core containing gallotannins (GCGs)’ against the α-glucosidase enzyme. The GCGs included ginnalins A, B and C (containing two, one, and one galloyl/s, respectively), maplexin F (containing 3 galloyls) and maplexin J (containing 4 galloyls). All of the GCGs were noncompetitive inhibitors of α-glucosidase and their interactions with the enzyme were further explored using biophysical and spectroscopic measurements. Thermodynamic parameters (by isothermal titration calorimetry) revealed a 1 : 1 binding ratio between GCGs and α-glucosidase. The binding regions between the GCGs and α-glucosidase, probed by a fluorescent tag, 1,1′-bis(4-anilino-5-naphthalenesulfonic acid), revealed that the GCGs decreased the hydrophobic surface of the enzyme. In addition, circular dichroism analyses showed that the GCGs bind to α-glucosidase and lead to loss of the secondary α-helix structure of the protein. Also, molecular modeling was used to predict the binding site between the GCGs and the α-glucosidase enzyme. This is the first study to evaluate the mechanisms of inhibitory activities of gallotannins containing a glucitol core on α-glucosidase.

 Please wait while we load your content...
Please wait while we load your content...