Synthesis of novel benzoxaborinin-4-ones and its application in indolin-2-ones synthesis using a Suzuki–Miyaura reaction protocol†
Abstract
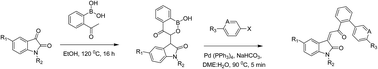
We herein discuss the synthesis of novel benzoxaborinin-4-one from substituted isatins and 2-acetyl phenylboronic acid. Furthermore, we have demonstrated the application of these boronic acids to synthesize indolin-2-ones (Z isomer) regioselectively using Suzuki–Miyaura reaction.

 Please wait while we load your content...
Please wait while we load your content...