pH sensitive smart gels of cetylpyridinium chloride in binary solvent mixtures: phase behaviour, structure and composition†
Abstract
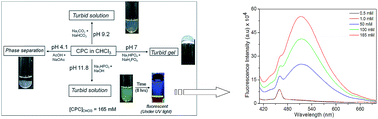
Cetylpyridinium chloride (CPC) gels from binary solvent mixtures of chlorinated solvents in the presence of water at a specific composition ratio have been studied. A transparent gel has been formed from CPC with dichloromethane, while a turbid gel with chloroform and a very weak opaque gel with carbon tetrachloride were observed in the presence of water. The CPC gel in a binary solvent mixture at a critical solvent composition of 3 : 1 v/v CHCl3 : H2O has been investigated as a function of pH between 4.1–11.8. The self-assembly of CPC and its morphology was found to be dependent on the solvent polarity/dielectric constant and pH of the medium. The absorption and emission characteristics of the CPC gel showed a significant response in a highly alkaline medium. The microstructure of the CPC gels in various chlorinated solvent combinations was proposed based on spectroscopic and microscopic investigations.

 Please wait while we load your content...
Please wait while we load your content...