Palladium-catalyzed C-7 alkenylation of indolines using molecular oxygen as the sole oxidant†
Abstract
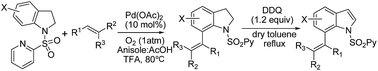
A general and efficient method for the intermolecular direct C-7-selective C–H alkenylation of indolines using palladium(II) as the catalyst and molecular oxygen as the sole oxidant has been developed. The reaction showed complete regio- and stereoselectivity. All products were E-isomers at the C-7 position, and no Z-isomers or other position substituted products could be detected. The approach also presented an efficient route for the synthesis of C-7 alkenylated indoles.

 Please wait while we load your content...
Please wait while we load your content...