Design, synthesis, biological evaluation and molecular docking of novel dabigatran derivatives as potential thrombin inhibitors†
Abstract
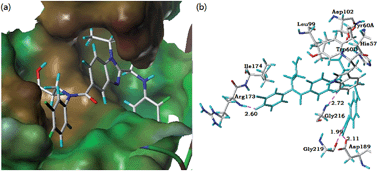
A series of dabigatran derivatives were designed and synthesized to discover effective thrombin inhibitors. All the target compounds were characterized by 1H NMR, 13C NMR and HRMS. These compounds were evaluated in vitro and showed potent anticoagulant activities against thrombin. Among these compounds, the ethyl group substitution at N-1 of benzimidazole exhibited better anticoagulant activity against thrombin. Especially, compound 10i showed an IC50 of 2.04 nM. Docking simulations demonstrated that compound 10i could bind tightly with the crystal structure of the thrombin active site. Based on the results obtained, compound 10i may act as a candidate compound for further development of direct thrombin inhibitors.

 Please wait while we load your content...
Please wait while we load your content...