Phase and structure development of spontaneously ambient-grown ZnO·xH2O and TiO2·xH2O nanostructures towards oxide single crystals
Abstract
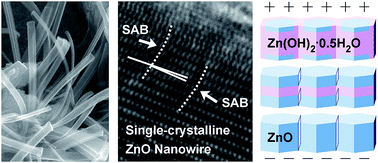
Stress-induced ZnO·xH2O and TiO2·xH2O nanocrystals were spontaneously grown on ZnO and TiO2 films in an ambient atmosphere based on a bond breaking–hydrolysis–reconstruction mechanism, without the use of any other precursors. The development of their phase and structure towards ZnO and TiO2 was in situ and ex situ studied. The formation of unstable near-amorphous belt-like orthorhombic ZnO·1.5H2O nanowires and partially crystalline column-like (pyramid top) monoclinic TiO2·2.5H2O nanorods was initiated in a high relative humidity of 98%, whereas more stable polycrystalline faceted orthorhombic ZnO·H2O nanowires and bamboo leaf-shaped monoclinic TiO2·0.4H2O nanoflakes were formed in 70% humidity. Upon receiving energy through in situ exposure to an electron beam or ex situ thermal annealing, the ZnO·xH2O and TiO2·xH2O transformed into hexagonal (wurtzite) ZnO and orthorhombic (brookite) TiO2, respectively. Exposure of non-polar TiO2 to an electron beam or annealing generated a polycrystalline structure. Exposure of polar ZnO to a low-energy electron beam caused the formation of aligned subgrains, while high-energy annealing yielded a single-crystalline structure, both with a longitudinal [10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] orientation, via a self-assembly process that involved nanocrystallite agglomeration, subgrain tilting and boundary elimination.
0] orientation, via a self-assembly process that involved nanocrystallite agglomeration, subgrain tilting and boundary elimination.

 Please wait while we load your content...
Please wait while we load your content...