Study on key step of 1,3-butadiene formation from ethanol on MgO/SiO2
Abstract
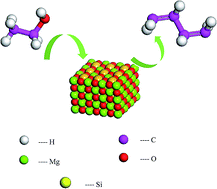
The structure and surface properties of the MgO/SiO2 catalyst were studied by both experimental characterization and the simulation method. The adsorption properties of ethanol on different MgO/SiO2 surfaces were researched by the density functional theory method and the result is that ethanol mainly adsorbed on MgO surface. The role of SiO2 in increasing the MgO crystal defects in the MgO/SiO2 has been obtained and the electronic properties of ethanol molecule before and after adsorption on the catalyst surfaces were compared. The initial step of ethanol dehydrogenation and the dehydration to 1,3-butadiene process, which is the dehydrogenation of ethanol to acetaldehyde reaction, on the flat sites, stepped sites and kinked sites was examined through the molecular simulation method. We investigated the preferable surface for the reaction of ethanol dissociation to an ethoxy group and the more active surface for the reaction of dehydrogenation of ethanol to acetaldehyde. The research results suggested that the stepped MgO surface is more active for the reaction of dehydrogenation of ethanol to acetaldehyde.

 Please wait while we load your content...
Please wait while we load your content...