Experimental and DFT study on the indium-mediated synthesis of benzophenones via arylstannanes†
Abstract
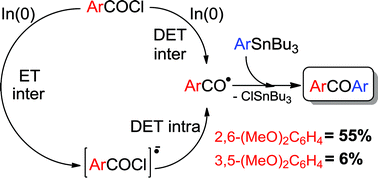
Experimental results of the solvent-free, indium-promoted reaction of aroyl chlorides with arylstannanes showed a narrow scope; its efficiency depends both on the extent of methylation in the latter and on the nature, number and position of the substituents in the former. With the purpose of explaining experimental results, a theoretical analysis with DFT methods was performed for a set of selected cases.

 Please wait while we load your content...
Please wait while we load your content...