Electrodeposit copper from alkaline cyanide-free baths containing 5,5′-dimethylhydantoin and citrate as complexing agents†
Abstract
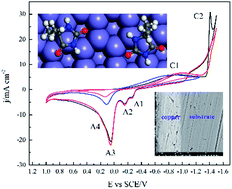
An alkaline cyanide-free bath containing 5,5′-dimethylhydantoin (DMH) and citrate, as complexing agents, was investigated and developed for copper electrodeposition on carbon steel substrate. The cathodic electrodeposition process was studied by electrochemical measurements including cyclic voltammetry, chronoamperometry, chronopotentiometry, and cathodic polarization on platinum and glass carbon disk electrodes. Copper layers were characterized with SEM for surface morphology, XRD for crystal structure, and three qualitative methods for adhesion evaluation. Based on the cyclic voltammetry analysis with various switching potentials the discharge processes were proven to have the typical two steps for solutions containing either DMH, or citrate, or both as complexing agents. The discharge processes of both copper–DMH and copper–citrate complexes are irreversible according to cyclic voltammetry with various scan rates, which combined with sample current voltammetry generates the kinetic parameters. Diffusion coefficients were calculated from chronoamperometry according to Conttrell equation. The nucleation and crystal growth processes on the glass carbon electrode, in these three solutions, show an agreement with the progressive nucleation process of SH models. Molecular dynamic simulations reveal that the plane of the heterocycle of all the four DMH molecules simulated is parallel to the Fe (111) surface and the interaction energy between various molecules and Cu (111) as well as Fe (111) surface were compared. DMH molecules adsorbed on the carbon steel electrode desorb at the initial stage of electrodeposition, which was determined from potential vs. time curves. The concentrations of DMH and K2CO3 as well as the value of pH have a crucial effect on the surface morphology, preferable crystal orientation, and the adhesion of copper layers electroplated on the carbon steel substrate. The electrodeposited copper layers had good adhesion with the carbon steel substrate and the average grain size was about 30 nm. The optimum bath is composed of 0.1 M CuSO4, 0.2 M DMH, 0.3 M citrate, and 0.3 M K2CO3 at pH 9–10.5 and 50 °C.

 Please wait while we load your content...
Please wait while we load your content...