One-pot synthesis of imine from benzyl alcohol and nitrobenzene on visible-light responsive CdS–TiO2 photocatalysts
Abstract
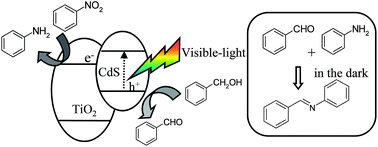
The one-pot synthesis of imine (N-benzylideneaniline) from benzyl alcohol and nitrobenzene was studied on the CdS–TiO2 photocatalyst under visible-light irradiation (λ > 420 nm) at 298 K. The photocatalytic activity strongly depends on the loadings of CdS on the TiO2, and the preferred amount of CdS was 15 wt%. Photo-electrochemical investigations were also incorporated to demonstrate the reaction mechanism: the CdS is photosensitized by visible-light irradiation, and an effective hole–electron separation is generated in the hetero-junction between the CdS and TiO2. It was also confirmed that the photocatalytic oxidation of benzyl alcohol proceeds over the CdS surface, while the hydrogenative reduction of nitrobenzene into aminobenzene proceeds over the TiO2 surface. Moreover, the production of imine proceeded efficiently by the successive condensation of benzaldehyde and aminobenzene on the catalyst surface under dark conditions.

 Please wait while we load your content...
Please wait while we load your content...