Probing the evolution of an Ar-BINMOL-derived salen–Co(iii) complex for asymmetric Henry reactions of aromatic aldehydes: salan–Cu(ii) versus salen–Co(iii) catalysis†
Abstract
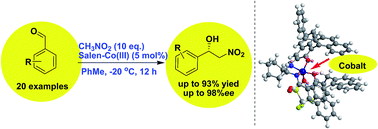
A new type of chiral salen–Co catalyst that features aromatic π-walls and an active Co(III) center has been developed for enantioselective Henry/nitroaldol reactions on the basis of salen–Cu catalysis. The asymmetric Henry reaction of aromatic aldehydes and nitromethane catalyzed by an Ar-BINMOL-derived salen–Co(III) complex was achieved with high yields (up to 93%) and excellent enantioselectivities (up to 98% ee). And more interestingly, it was supposed that either salan–Cu(II) or salen–Co(III) complex-catalyzed Henry reaction was an ideal model reaction for providing direct evidence of noncovalent interaction due to the distinguishable ortho-substituted aromatic aldehydes from meta- or para-substituted benzaldehydes in terms of enantioselectivities and yields.

 Please wait while we load your content...
Please wait while we load your content...