Design and synthesis of optically pure 3-aryl-6-methyl-2-thioxotetrahydropyrimidin-4(1H)-ones as anti-prostate cancer agents†
Abstract
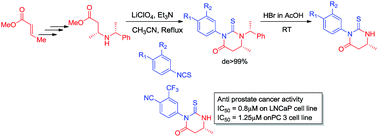
3-Aryl-6-methyl-2-thioxotetrahydropyrimidin-4(1H)-ones were proposed as a new class of anti-prostate cancer agents on the basis of molecular modeling studies. Stereoselective synthesis of 3-aryl-6-methyl-2-thioxotetrahydropyrimidin-4(1H)-one derivatives was achieved by chiral induction employing (R)/(S)-α-methyl benzylamine and subsequent debenzylation with HBr in AcOH afforded the desired enantiomers in good yields. The compounds were screened in vitro against prostate cancer cell lines, PC-3 and LNCaP and the most potent derivatives were identified.

 Please wait while we load your content...
Please wait while we load your content...