Sol–gel auto-combustion synthesis of Ni–CexZr1−xO2 catalysts for carbon dioxide reforming of methane†
Abstract
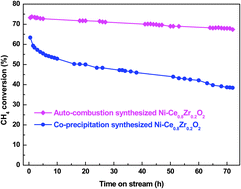
Carbon dioxide reforming of methane (methane dry reforming) over Ni–Ce0.8Zr0.2O2 catalysts prepared by a sol–gel auto-combustion method and a conventional co-precipitation method were comparatively studied. We show that sol–gel auto-combustion is very promising for preparing thermal stable homogeneous mixed metal oxide catalysts. The auto-combustion synthesized catalyst exhibited higher initial activity and stability due to its smaller Ni crystalline size and intimate interaction between Ni and Ce0.8Zr0.2O2. In contrast, the co-precipitated catalyst showed poor activity and deactivated rapidly. The rapid deactivation was caused by a higher graphitization degree of the deposited carbon over co-precipitated catalyst with larger Ni crystalline size. We also found that the physico-chemical properties and catalytic activity of sol–gel auto-combustion synthesized catalysts were closely related to the metal nitrate (MN)/citric acid (CA) ratio. High MN/CA ratio led to more violent combustion behaviour and an accordingly higher degree of crystallization of the synthesized catalyst. In contrast, a low MN/CA ratio resulted in more carbon species residues and poor catalytic performance. The Ce/Zr ratio also had a profound influence on the phase structure, reducibility, oxygen vacancies and catalytic performance of Ni–Ce0.8Zr0.2O2 catalysts. Ni–Ce0.8Zr0.2O2 catalyst with cubic phase exhibited the best catalytic performance because of high reducibility, high Ni dispersion and strong Ni-CexZr1−xO2 interaction, and considerable amounts of oxygen vacancies.

 Please wait while we load your content...
Please wait while we load your content...