Mechanism and origins of gold-catalyzed domino cyclization to spiroindolines: the role of periplanar cooperation and hydrogen bonding interactions†
Abstract
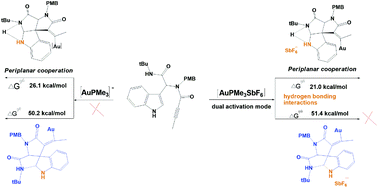
The detailed mechanism and origins of gold-catalyzed domino cyclization to indoloazocines by density functional theory are systematically studied. The energy profiles for [Au(PMe3)]+ and [Au(PMe3)SbF6] were investigated. Specifically, the gold-counterion dual catalysis mechanism was the most plausible mechanism for domino cyclization to spiroindolines because of its periplanar cooperation, small activation energy, and hydrogen bonding interactions in the transition states (TS) and intermediates. Based on the Curtin–Hammett principle, the calculated activation energy of 21.0 kcal mol−1 was the rate-determining step for the overall reaction. Besides, the energy profiles for three different models, i.e. catalysts with OTf−counterion, BF4− counterion, and THF solvent, were investigated to confirm the role of hydrogen bonding interactions in the cooperative dual catalysis mechanism. Thus, the obtained theoretical results not only well rationalize the experimental results but also provide insights into the details of the domino cyclization.


 Please wait while we load your content...
Please wait while we load your content...