Total synthesis of antiallergic bicyclic peptide seongsanamide A†
Abstract
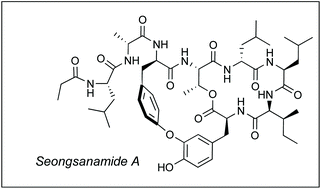
The first total synthesis of antiallergic depsipeptide seongsanamide A has been achieved and also the relative and absolute stereochemistry of the natural product has been confirmed. Highlights of the convergent route include the use of Miyuara borylation, Chan–Evans–Lam coupling for the effective assembly of the isodityrosine subunit and the identification of an effective macrocyclization site in very high conversion. The longest linear sequence leading to seongsanamide A was 12 steps, with an overall yield of 12.7%.


 Please wait while we load your content...
Please wait while we load your content...