Merging Cu-catalysed C–H functionalisation and intramolecular annulations: computational and experimental studies on an expedient construction of complex fused heterocycles†
Abstract
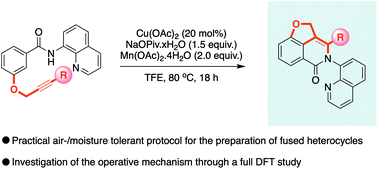
Intramolecular annulation reactions provide a powerful opportunity to access complex heterocyclic compounds with higher complexity than intermolecular conversions. This report details how, previously unknown fused dihydrobenzofuran-isoquinolone compounds, exhibiting an unusually strained shared aromatic unit, can be readily obtained from simply prepared benzamide derivatives bearing a tethered alkyne moiety, using copper C–H bond functionalisation catalysis. The mechanism has been proposed based on detailed DFT and topological analysis studies, and shows that the two key heterocycles are formed during distinct mechanistic steps; the dihydrobenzofuran arises from a migratory insertion and the isoquinolone from the following reductive elimination, resulting in an efficient Double Annulation Reaction (DAR). Actually, the results present an unprecedented migratory insertion of alkynes with benzamides when using copper as catalyst with the 8-aminoquinoline directing group and also study why the intermolecular variant is not operative.


 Please wait while we load your content...
Please wait while we load your content...