Exploration of 1,3-dipolar cycloaddition reactions to construct the core skeleton of Calyciphylline A-type alkaloids†
Abstract
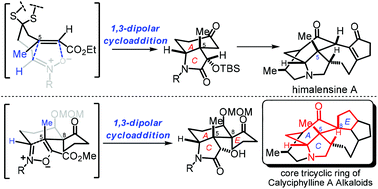
Nitrone induced 1,3-dipolar [3 + 2] cycloadditions were studied to construct the core structure of Calyciphylline A-type Daphniphyllum alkaloids. This approach is capable of installing the cis-hydroindole A–C ring as well as the spiro-A–C–E ring with the all-carbon quaternary centers at C-5 and C-8, and has been successfully used in the total synthesis of himalensine A. It also lays the foundation for the synthesis of challenging Calyciphylline A-type alkaloids, such as daphniyunnine A.


 Please wait while we load your content...
Please wait while we load your content...