General approach to 2-fluoroalkyl 1,3-azoles via the tandem ring opening and defluorinative annulation of N-fluoroalkyl-1,2,3-triazoles†
Abstract
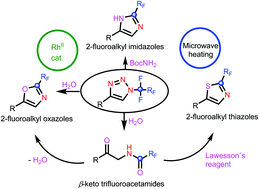
Rhodium-catalyzed transformation of N-fluoroalkyl-1,2,3-triazoles in the presence of tert-butyl carbamate or water provided an efficient route to 2-fluoroalkyl imidazoles and 2-fluoroalkyl oxazoles, respectively. The treatment of N-fluoroalkyl-1,2,3-triazoles with excess water gave fluoroalkylated ketamides which were cyclized in situ to 2-fluoroalkyl oxazoles or thiazoles. Trifluoromethyl- and other difluoroalkyl-substituted heterocycles were obtained by the present method. The mechanism of this cascade transformation involving carbene insertion, HF elimination and cyclization was proposed.


 Please wait while we load your content...
Please wait while we load your content...