β-Sulfonylation of α-bromoenals enabled by N-heterocyclic carbene catalysis†
Abstract
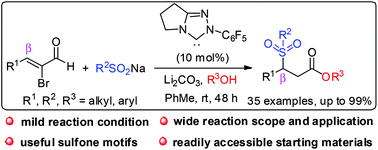
An N-heterocyclic carbene (NHC)-catalyzed three-component tandem β-sulfonylation/esterification of α-bromoenals was developed. This protocol offers a simple and efficient pathway to access a wide range of functionalized alkyl sulfone esters that cannot be obtained through direct β-sulfonylation of internal α,β-unsaturated esters. The preference of β-sulfonylation over direct esterification of β-activated α,β-unsaturated acylazoliums and use of a weaker base were key to the success of this transformation.


 Please wait while we load your content...
Please wait while we load your content...