Highly convergent modular access to poly-carbon substituted cyclopropanes via Cu(i)-catalyzed three-component cyclopropene carboallylation†
Abstract
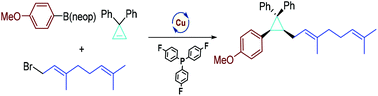
We report herein the first example of conjunctive C–C cross-coupling of cyclopropenes enabled by a Cu-catalyzed three-component reaction of organoboron, cyclopropene and allylic bromide, which features a modular, stereoselective assembly of poly-carbon substituted cyclopropanes. Preliminary studies showed that the reaction can be extended to benzylation and made enantioselective with C2-symmetrical bisphosphine ligands.


 Please wait while we load your content...
Please wait while we load your content...