Synthesis of chromans and kinetic resolution of 2-aryl-3-nitro-2H-chromenes via the NHC-bound azolium homoenolate pathway†
Abstract
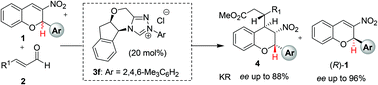
Beyond the tandem oxa-Michael strategy, an N-heterocyclic carbene-catalyzed kinetic resolution approach to access highly diastereo- and enantioenriched 2-aryl-3-nitro-chromane derivatives has been developed. An efficient strategy for the kinetic resolution of racemic 2-aryl-3-nitro-2H-chromenes was also successfully achieved. This catalytic kinetic resolution method allows the synthetically valuable chiral scaffolds, chromane and 2H-chromene, to be accessed in a single catalytic and asymmetric transformation, through the NHC-bound azolium homoenolate pathway.


 Please wait while we load your content...
Please wait while we load your content...