Steric hindrance classified: treatment of isothiocyanatoallene with secondary amines bearing bulky substituents to generate 2-aminothiazoles†‡
Abstract
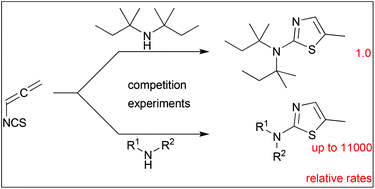
Twenty secondary amines, bearing two secondary, two tertiary, or a secondary and a tertiary alkyl group or a neopentyl group, were treated with allenyl isothiocyanate to prepare the corresponding 2-dialkylamino-5-methylthiazoles. The relative rates of these reactions, which were strongly dependent on the steric demand of the N-alkyl groups, were measured by using competition experiments and analysis by 1H NMR spectroscopy. Not only steric, but also some electronic effects on the nucleophilicity of the dialkylamines were discussed.


 Please wait while we load your content...
Please wait while we load your content...