Asymmetric total syntheses of callistrilones B, G and J†
Abstract
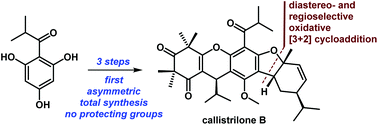
A very concise approach for the first asymmetric and scalable total syntheses of callistrilones B, G and J has been developed. These syntheses proceeded in only 3–4 steps from the commercially available (−)-α-phellandrene (8), without the need for protecting groups. The key feature of the syntheses was a biomimetic, diastereo- and regioselective oxidative [3 + 2] cycloaddition.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...