Total synthesis of C19-diterpenoid alkaloid: construction of a functionalized ABCDE-ring system†
Abstract
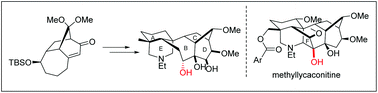
A synthetic approach for the ABCDE ring system of methyllycaconitine starting from a BCD tricycle precursor 14 was described. The synthesis features a useful strategy for the full functionalization of ring B, a 1,7-enyne reductive radical cyclization for the construction of ring A, and a straightforward double condensation for installation of the piperidine ring E.


 Please wait while we load your content...
Please wait while we load your content...