Catalyst-controlled synthesis of 4-amino-isoquinolin-1(2H)-one and oxazole derivatives†
Abstract
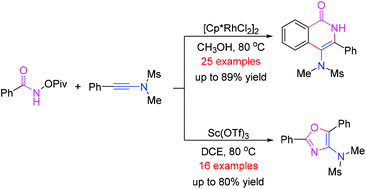
A facile synthetic method to access 4-amino-isoquinolin-1(2H)-one and oxazole derivatives was disclosed in this paper. The reaction of N-(pivaloyloxy)-amides with ynamides produced 4-amino-isoquinolin-1(2H)-ones in good yields in the presence of Cp*Rh(III) catalyst through a C–H bond functionalization. Using Sc(OTf)3 as the catalyst, oxazole derivatives were obtained in good yields from the same reaction. This protocol features good functional group tolerance and excellent regioselectivity.
- This article is part of the themed collections: Celebrating 70 Years of Shanghai Institute of Organic Chemistry and Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...