Highly efficient and versatile synthesis of α,α-difluoro-γ-lactams via aminodifluoroalkylation of alkenes†
Abstract
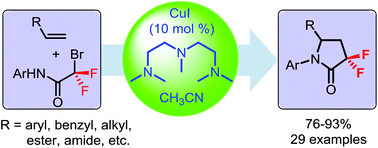
A novel method for the synthesis of α,α-difluoro-γ-lactam derivatives has been developed using a copper/amine catalyst via a tandem radical cyclization pathway. This protocol provides a convenient and straightforward strategy for the rapid construction of various 3,3-difluoro-γ-lactam moieties from easily available alkenes and N-aryl bromodifluoroacetamide as starting materials under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...