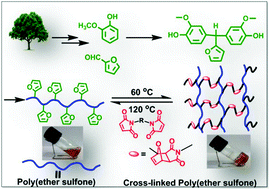
Partially bio-based aromatic poly(ether sulfone)s bearing pendant furyl groups: synthesis, characterization and thermo-reversible cross-linking with a bismaleimide†
Abstract
A fully bio-based bisphenol bearing pendant furyl group, viz., 4,4′-(furan-2-ylmethylene)bis(2-methoxyphenol) (BPF) was synthesized by base-catalyzed condensation of furfural with guaiacol. New partially bio-based (co)poly(ether sulfone)s bearing pendant furyl groups were synthesized via aromatic nucleophilic substitution polycondensation of BPF and various mixtures of BPF and bisphenol-A with bis(4-fluorophenyl) sulfone in N,N-dimethylacetamide as a solvent. (Co)poly(ether sulfone)s showed inherent viscosities in the range 0.92–1.47 dL g−1 and number average molecular weights 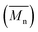 , obtained from GPC, were in the range 91 300–131 000 g mol−1. (Co)poly(ether sulfone)s could be cast into tough, transparent and flexible films from chloroform solutions. (Co)poly(ether sulfone)s showed 10% weight loss in the temperature range 431–481 °C and DSC showed Tg in the range 179–190 °C. Tensile strength, Young's modulus and elongation at break, obtained from tensile testing of (co)poly(ether sulfone)s containing pendant furyl groups, were in the range 76.7–83.8 MPa, 1.02–1.18 GPa and 21.4–89.7%, respectively. Most interestingly, pendant furyl groups in (co)poly(ether sulfone)s provide reactive sites for click modification and cross-linking via Diels–Alder reaction with maleimides and bismaleimides, respectively. Cross-linked (co)poly(ether sulfone) was prepared via Diels–Alder reaction of a representative (co)poly(ether sulfone) containing pendant furyl groups with 1,1′-(methylenedi-1,4-phenylene)bismaleimide (BMI) at 60 °C. The formation of cross-linked poly(ether sulfone) was demonstrated by gelation tests, solubility tests and DSC. The cross-linked copoly(ether sulfone) prepared using BMI showed enhanced tensile strength and Young's modulus compared to parent copoly(ether sulfone) and was recycled two times with retention of mechanical properties.
, obtained from GPC, were in the range 91 300–131 000 g mol−1. (Co)poly(ether sulfone)s could be cast into tough, transparent and flexible films from chloroform solutions. (Co)poly(ether sulfone)s showed 10% weight loss in the temperature range 431–481 °C and DSC showed Tg in the range 179–190 °C. Tensile strength, Young's modulus and elongation at break, obtained from tensile testing of (co)poly(ether sulfone)s containing pendant furyl groups, were in the range 76.7–83.8 MPa, 1.02–1.18 GPa and 21.4–89.7%, respectively. Most interestingly, pendant furyl groups in (co)poly(ether sulfone)s provide reactive sites for click modification and cross-linking via Diels–Alder reaction with maleimides and bismaleimides, respectively. Cross-linked (co)poly(ether sulfone) was prepared via Diels–Alder reaction of a representative (co)poly(ether sulfone) containing pendant furyl groups with 1,1′-(methylenedi-1,4-phenylene)bismaleimide (BMI) at 60 °C. The formation of cross-linked poly(ether sulfone) was demonstrated by gelation tests, solubility tests and DSC. The cross-linked copoly(ether sulfone) prepared using BMI showed enhanced tensile strength and Young's modulus compared to parent copoly(ether sulfone) and was recycled two times with retention of mechanical properties.


 Please wait while we load your content...
Please wait while we load your content...