Synthesis of polymer-linked copper(i) bis(N-heterocyclic carbene) complexes of linear and chain extended architecture†
Abstract
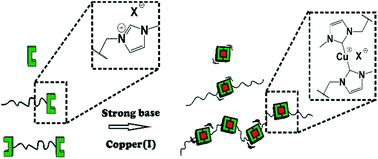
Copper(I) bis(N-heterocyclic carbene) (NHC) complexes can be used as catalysts for a wide range of different reactions, like hydrosilylation, CH-activation or copper(I)-catalyzed alkyne/azide cycloaddition (CuAAC) reactions. In this paper a novel synthetic approach towards poly(styrene) (PS)-based copper(I) bis(NHC) complexes with linear and chain extended different architectures and their defined activation for CuAAC by mechanical force is reported. The NHC precursor polymers are synthesized via living RAFT polymerization to ensure defined polymer compositions achieving the N-methylimidazolium functionalization either by modified chain transfer agents (CTA) or via efficient post-polymerization thio-bromo click reactions. Subsequent deprotonation by NaOtBu and coordination to a central copper(I) were used to generate low molecular weight, polymeric linear and chain extended copper(I) bis(NHC) complexes bearing more than one copper(I) center with molecular weights up to 50 000 g mol−1. A mechanochemical activation of these complexes is demonstrated in bulk quantifying the catalytic activity in a fluorogenic CuAAC click reaction with conversions up to 44%, which allows the use of these polymers as stress-sensors.


 Please wait while we load your content...
Please wait while we load your content...