Environmentally-friendly processing of thermosets by two-stage sequential aza-Michael addition and free-radical polymerization of amine–acrylate mixtures
Abstract
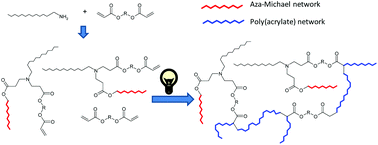
A new dual-curing, solvent-free process is described for the preparation of tailor-made materials from off-stoichiometric amine–acrylate formulations. The first stage reaction is a self-limiting click aza-Michael addition between multifunctional amine and acrylate monomers with an excess of acrylate groups. The second stage reaction is a photoinduced radical polymerization of the unreacted acrylate groups. By selecting the structure of the monomers and the stoichiometry of the formulations, mechanical and thermal characteristics of the intermediate and final materials can be tuned. The materials obtained after the first curing stage can be gelled or ungelled and loosely or tightly crosslinked at the end of the second curing stage. The methodology used allows to obtain storable and processable intermediate polymers and final networks with optimum properties for different applications. The presence of amines in the reaction medium overcomes the intrinsic oxygen inhibition of acrylate free-radical polymerizations, resulting in a quasi complete cure.


 Please wait while we load your content...
Please wait while we load your content...