Amorphous and crystalline blends from polytyrosine and pyridine-functionalized anthracene: hydrogen-bond interactions, conformations, intramolecular charge transfer and aggregation-induced emission†
Abstract
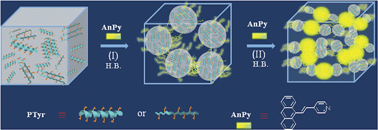
A new pyridine-terminated fluorophore of (E)-4-(2-(anthracen-9-yl)vinyl)pyridine (AnPy) with intramolecular charge transfer (ICT) and aggregation-induced emission (AIE) properties was synthesized and was blended with different amounts of polytyrosine (PTyr) through preferable hydrogen-bond (H-bond) interactions. In blends of a low AnPy content, the rigid PTyr peptide chains serve as templates to H-bond to AnPys, imposing rotational restriction and reinforcing the AIE-related emission intensity of AnPys, resulting in amorphous blends with the observed glass transitions dependent on the composition of the blends. In contrast, when large amounts of AnPys were added, excess AnPys will form new crystals, in between the amorphous regions, constituted of the near parallel dimers of AnPys. With the hampered molecular rotation, the parallel dimers of AnPys in the highly AnPy-loaded blends emit strongly with intensity much higher than those for the amorphous blends. In this study, conformations of the blends and the degree of restricted molecular rotation were assessed in order to correlate with the AIE-related fluorescence behaviour.

 Please wait while we load your content...
Please wait while we load your content...