Water soluble multiarm-polyethylene glycol–betulinic acid prodrugs: design, synthesis, and in vivo effectiveness†
Abstract
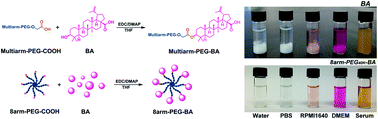
Betulinic acid (BA) is a new type of cancer-fighting drug, but it is limited by its low water solubility and relatively short half-life in clinical applications. To overcome the shortcomings, BA prodrugs were prepared by using multiarm-polyethylene glycol linkers. The prodrugs exhibited high drug loading capacity (3.26–11.81 wt%), high water solubility (290–750 fold of free BA), and excellent in vitro anticancer activity. Subsequent tumor xenograft assays demonstrated the superior therapeutic effect of BA prodrugs on inhibition of tumor growth compared with free BA. Multiple intravenous injection of BA prodrugs equivalent to 10 mg of BA per kg resulted in the decrease of an established implanted murine Lewis lung carcinoma (percent tumor growth inhibition after treatment on day 20, 72.1–90.7%) in mice. These results strongly supported that the BA prodrugs are promising for cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...