Practical polymerization of functionalized lactones and carbonates with Sn(OTf)2 in metal catalysed ring-opening polymerization methods†
Abstract
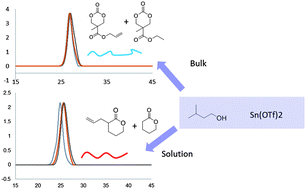
We report the synthesis of functionalized polycarbonates and polyesters employing stannous(II) trifluoromethane sulfonate [Sn(OTf)2] in homo and copolymerizations with monomers of the valerolactone family such as

 Please wait while we load your content...
Please wait while we load your content...