Kinetics of benzophenone ketyl free radicals recombination in a polymer: reactivity in the polymer cage vs. reactivity in the polymer bulk
Abstract
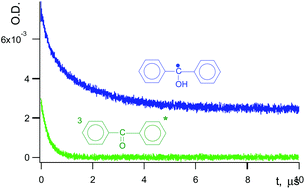
The decay kinetics of intermediates produced under photolysis of benzophenone (B) dissolved in soft rubber poly(ethylene-co-butylene) films (abbreviated as E) was studied by ns laser flash photolysis in the temperature range of 263–313 K. We monitored decay kinetics of the triplet state of 3B* and of benzophenone ketyl free radical BH˙. The fast exponential decay of 3B* (life-time τT ≈ 200 ns) is accompanied by hydrogen atom abstraction from E with a formation of BH˙ and a polymer free radical R˙. Decay of 3B* was followed by decay of BH˙ in the polymer cage (geminate recombination) with τc ≈ 1 μs. Cage recombination in turn was followed by a decay of BH˙ in the polymer bulk (τb ≈ 100 μs). Fortunately, all three processes are separated in time. Both cage and bulk reactions were decelerated by the application of magnetic field (MF) of 0.2 T by approximately 20%. Geminate recombination was fit to the first-order kinetic law, and recombination in the solvent bulk fits well to the second-order law. Both geminate recombination and recombination in the solvent bulk are predominantly a reaction between BH˙ and R˙. It was assumed that the reaction radius ρ12 and a mutual diffusion coefficient D12 of BH˙ and R˙ are the same for the cage and bulk recombination, respectively. This led to an estimation of ρ12 = 3.3 nm and D12 = 1 × 10−7 cm2 s−1. These values are discussed. We obtained activation energy, Eact, equal to 6 kcal mol−1 and 7 kcal mol−1 for cage decay and for recombination in the polymer bulk, respectively. These Eact coincide with each other within experimental error of their determination (±0.5 kcal mol−1). This indicates the same diffusion character in the cage and in the polymer bulk. It was demonstrated that an exponential model of cage effect sufficiently describes the obtained experimental data in rubber.

 Please wait while we load your content...
Please wait while we load your content...