Direct and water-mediated excited state intramolecular proton transfer (ESIPT) from phenol OH to carbon atoms of extended ortho-substituted biaryl systems†‡
Abstract
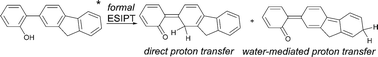
In our continuing studies of the generality and mechanistic details of a new type of excited state intramolecular
- This article is part of the themed collection: Photodynamic therapy and photodetection

 Please wait while we load your content...
Please wait while we load your content...