Sandmeyer reactions. Part 7.1 An investigation into the reduction steps of Sandmeyer hydroxylation and chlorination reactions
Abstract
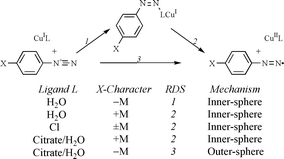
For Sandmeyer hydroxylation and chlorination in aqueous solution, the reduction steps have been investigated by means of correlation analyses of the effects of diazonium ion substitution on the rates of reduction. For simple hydroxylation, a change of behaviour between diazonium ions substituted by electron donor groups and those substituted by electron acceptor groups is interpreted as a change within an inner-sphere process from rate-determining electron transfer to rate-determining association of the reactants. By contrast, for citrate-promoted hydroxylation, a similar change in behaviour may be interpreted as a change between inner- and outer-sphere electron transfers. For chlorination, there is no mechanistic variation within the range of substituents examined but the pattern of behaviour is consistent with an inner-sphere mechanism. The various patterns of behaviour are rationalised in terms of the effects of diazonium ion substitution and catalyst ligation on the reduction potentials and self-exchange rates of the various reacting redox couples. Comparative correlation analyses of reductions and other electrophilic reactions of diazonium ions are used to support the arguments advanced in respect of Sandmeyer reduction steps. It is suggested that the CuI reductants react via a nucleophilic bridging ligand at the diazonium Nβ to give transient Z-adducts which are the precursor complexes and that activation for electron transfer involves rotation about the N–N bond.

 Please wait while we load your content...
Please wait while we load your content...