Oxidation of benzyl alcohol by pyridinium dichromate in acetonitrile. Using the para/meta ratio of substituent effects for mechanism elucidation†
Abstract
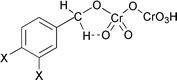
Rate constants were measured for the oxidation reaction of ![[small lambda, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cc.gif) = 1.09 ± 0.05 for the para/meta ratio of inductive or Electra effects is obtained and negative Hammett reaction constants decreasing in magnitude with increasing temperature are found. A mechanism implicating the prior acid-catalysed formation of neutral benzyl hydrogen dichromate ester followed by intramolecular
= 1.09 ± 0.05 for the para/meta ratio of inductive or Electra effects is obtained and negative Hammett reaction constants decreasing in magnitude with increasing temperature are found. A mechanism implicating the prior acid-catalysed formation of neutral benzyl hydrogen dichromate ester followed by intramolecular

 Please wait while we load your content...
Please wait while we load your content...