Novel aryltriazole acyclic C-azanucleosides as anticancer candidates†
Abstract
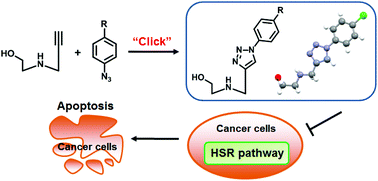
Nucleoside analogues represent an important class of drug candidates. With the aim of searching for novel bioactive nucleosides, we developed an efficient synthetic way to construct a series of aryl 1,2,3-triazole acyclic C-azanucleosides via Huisgen 1,3-dipolar cycloaddition. The aryl 1,2,3-triazole motifs within these azanucleosides showed coplanar features, suggesting they could act as surrogates for large planar aromatic systems or nucleobases. Moreover, several aryltriazole acyclic C-azanucleosides bearing long alkyl chains exhibited potent antiproliferative activity against various cancer cell lines via induction of apoptosis. Most interestingly, the lead compound significantly down-regulated the key proteins involved in the heat shock response pathway, representing the first anticancer acyclic azanucleoside with such a mode of action. These novel aryl 1,2,3-triazole cyclic C-azanucleosides therefore serve as promising paradigms for further exploring anticancer drug candidates.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...