Structural isomers of saligenin-based β2-agonists: synthesis and insight into the reaction mechanism†
Abstract
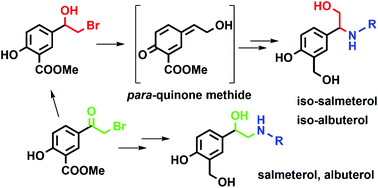
Salmeterol and albuterol are well-known β2-adenoreceptor agonists widely used in the treatment of inflammatory respiratory diseases, such as bronchial asthma and chronic obstructive pulmonary disease. Here we report the preparation of structural isomers of salmeterol and albuterol, which can be obtained from the same starting material as the corresponding β2-agonists, depending on the synthetic approach employed. Using 1D and various 2D NMR measurements, we determined that the structure of prepared isomers holds the β-aryl-β-aminoethanol moiety, in contrast to the α-aryl-β-aminoethanol moiety found in salmeterol and albuterol. We investigated the reaction of β-halohydrin and amines responsible for the formation of β-aryl-β-amino alcohol – both experimentally and using computational methods. The structure of β-halohydrin with the methyl salicylate moiety imposes the course of the reaction. The solvent plays a relevant, yet ambiguous role in the direction of the reaction, while the strength of the base influences the reaction yield and isomer ratio in a more evident way. Using computational methods, we have shown that the most probable reaction intermediate responsible for the formation of the unexpected isomer is the corresponding para-quinone methide, which can be formed due to phenol present in the methyl salicylate moiety. After successful preparation of albuterol and salmeterol isomers, we tested their inhibition potency to human acetylcholinesterase (AChE) and usual and atypical butyrylcholinesterase (BChE). Kinetic studies revealed that both isomers are low-potency reversible inhibitors of human cholinesterases.


 Please wait while we load your content...
Please wait while we load your content...