In situ air oxidation and photophysical studies of isoquinoline-fused N-heteroacenes†
Abstract
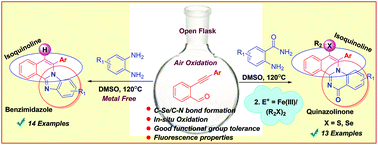
An efficient, metal free and environment friendly synthesis of isoquinoline-fused benzimidazole has been developed via in situ air oxidation. Also, syntheses of isoquinoline-fused quinazolinone heteroacenes were successfully achieved. The synthesized isoquinoline-fused benzimidazole and isoquinoline-fused quinazolinone derivatives showed λmax, Fmax and Φf values in the ranges 356–394 nm, 403–444 nm and 0.063–0.471, respectively, in CHCl3.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...